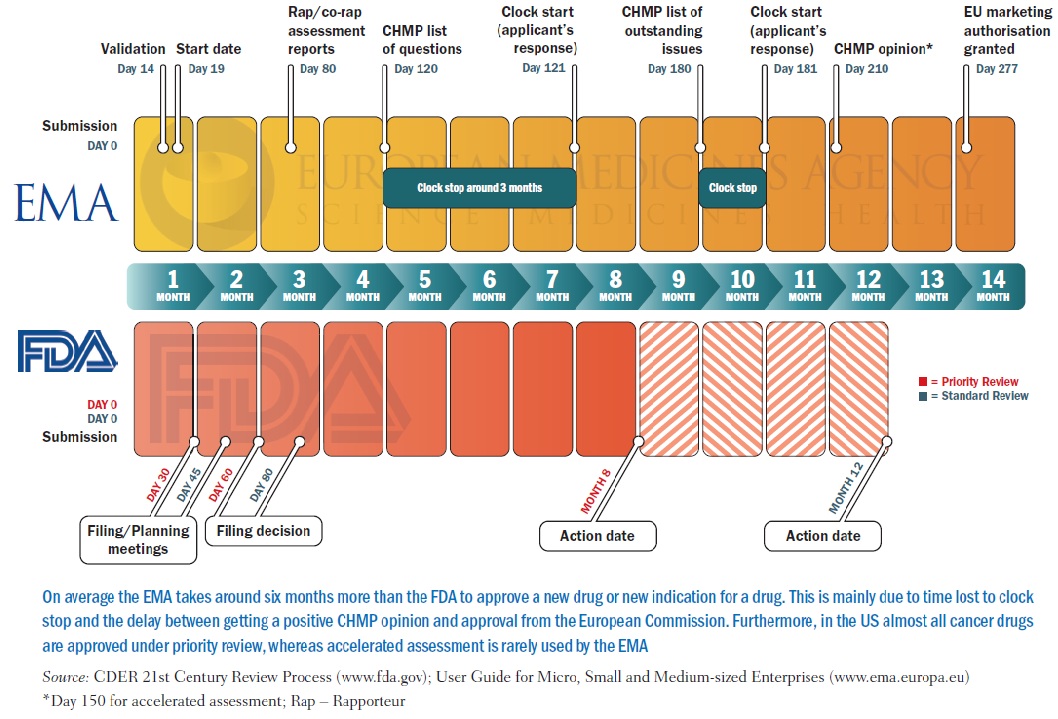
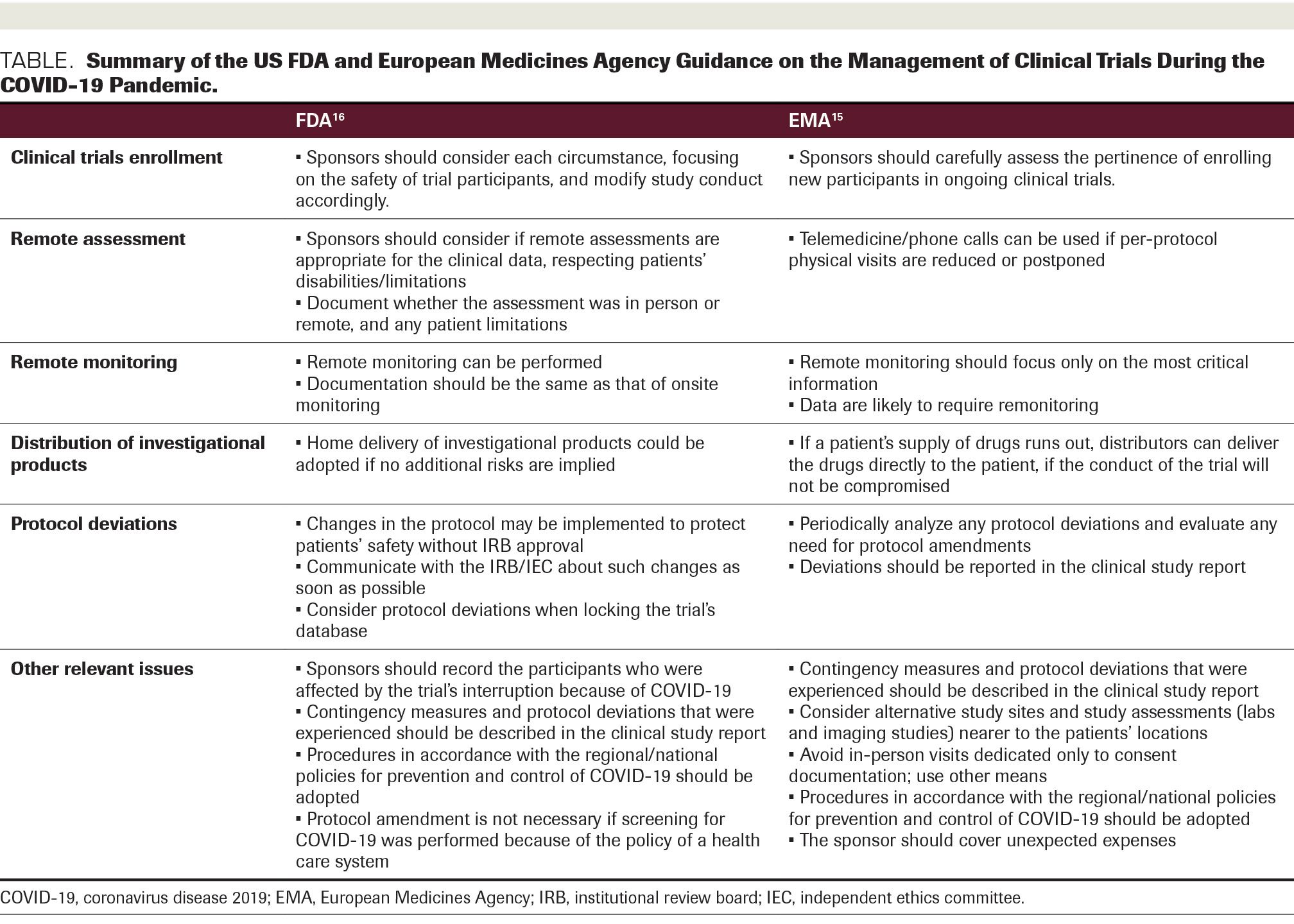
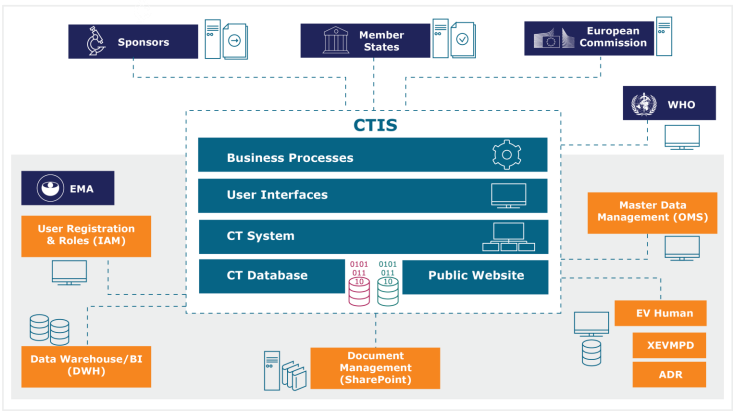
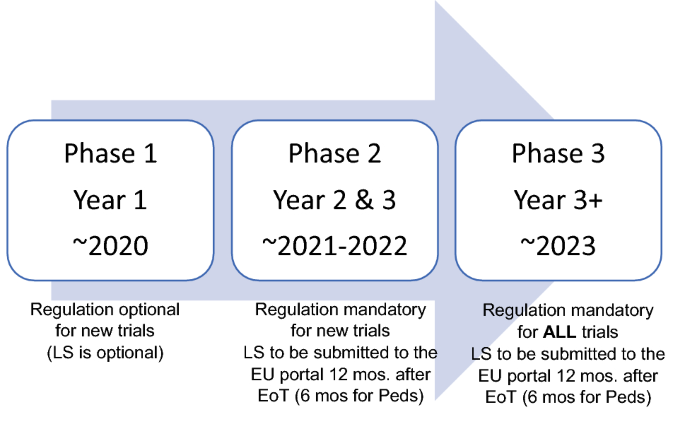
Characteristics of Single Pivotal Trials Supporting Regulatory Approvals of Novel Non‐orphan, Non‐oncology Drugs in the European Union and United States from 2012−2016 - Morant - 2019 - Clinical and Translational Science - Wiley Online Library

Comparison of regulatory pathways for the approval of advanced therapies in the European Union and the United States - Cytotherapy

EU Medicines Agency on Twitter: "In a letter published today, @EU_Commission, #EMA and the Heads of Medicines Agencies remind all sponsors of #ClinicalTrials conducted in the 🇪🇺 to make results of concluded

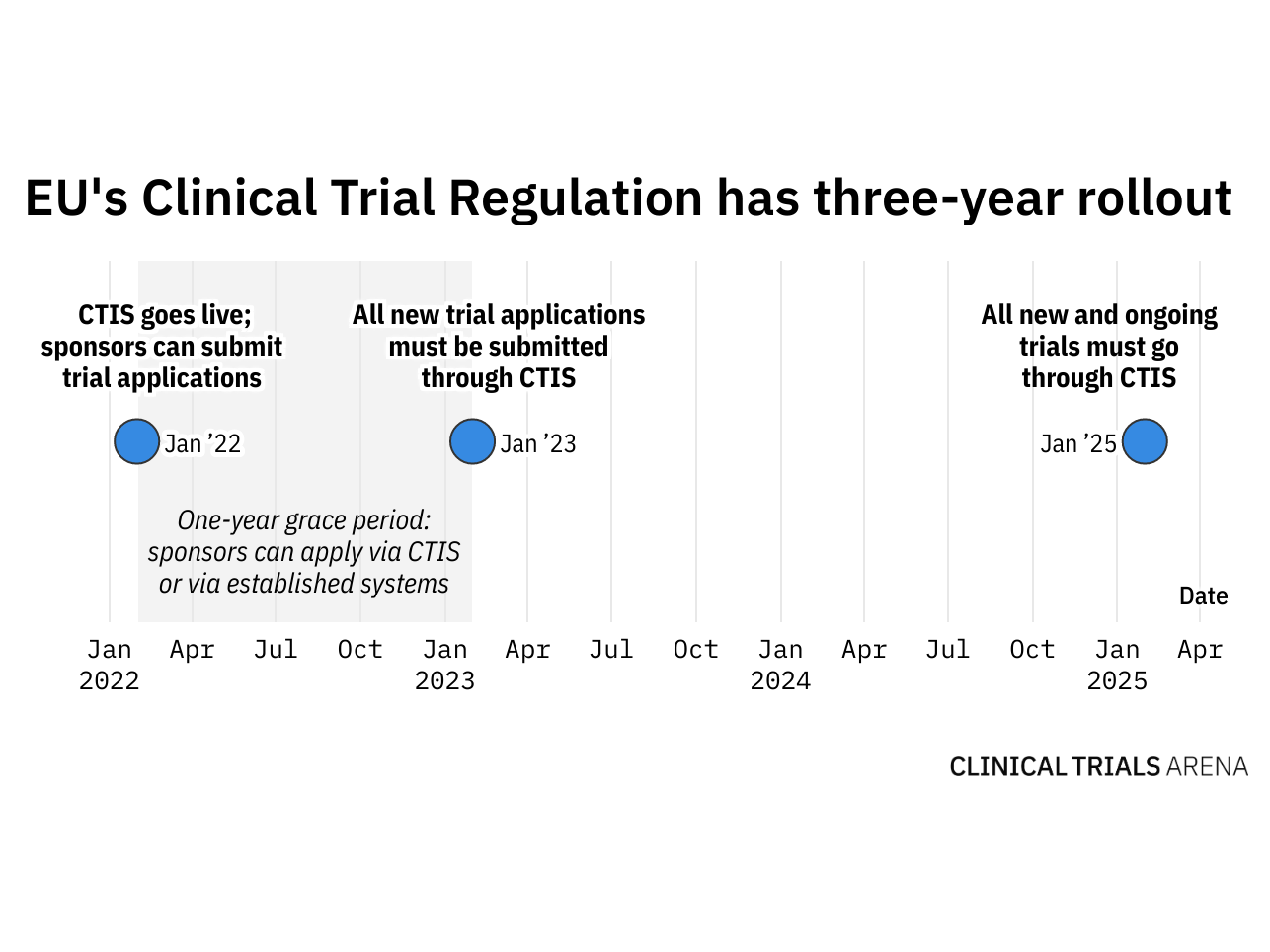
Clinical Trial Regulation Update - PharSafer® - Specialists in Global Clinical and Post Marketing Drug Safety
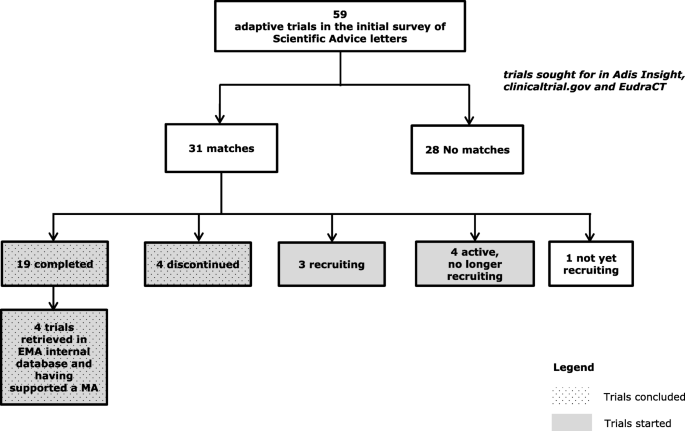
Adaptive designs in clinical trials: from scientific advice to marketing authorisation to the European Medicine Agency | Trials | Full Text